PhysicsBowl 2017 Q19
- keshprad

- Apr 9, 2020
- 1 min read
19) In this question, we are given some of the conditions of an ideal gas. We want to solve for the pressure of the gas. We need to use the ideal gas law to solve this problem.
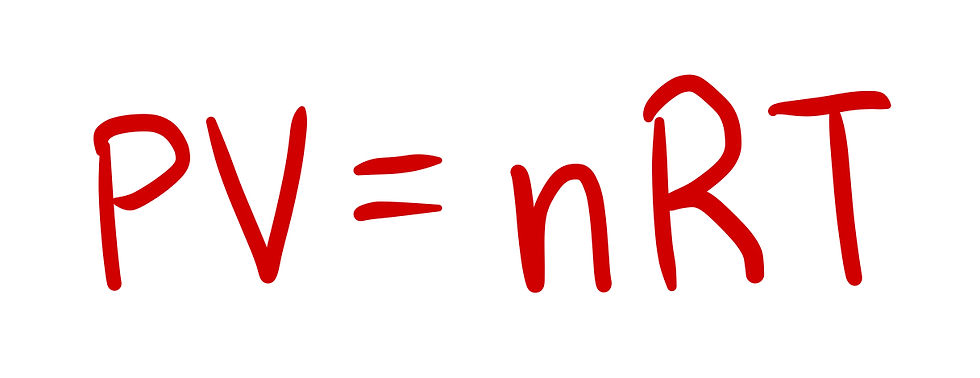
Notice that the volume is given in liters. If we take a look at the PhysicsBowl constants sheet, we will see that there are two values for R, the universal gas constant. Looking at the units for R, we see that only one of them are is liters, so we will use R as 8.21*10^-2 (L*atm)/(mol*K).
We also need to ensure that we use the temperature in Kelvin. Then, we can solve for the pressure.

Finally, we need to convert the pressure to units of Pascals.

Answer: B



Comments